Introduction: Hyperleukocytosis and leukostasis in acute leukemia are associated with high risks of organ failure and early death. The best management approach for hyperleukocytosis and the role of leukapheresis are poorly defined due to the lack of high quality evidence. We sought to study beliefs and patterns of practice in the management of hyperleukocytosis and leukapheresis among providers in North America.
Methods: We developed a 38-question survey to query providers caring for acute leukemia patients with hyperleukocytosis. The survey was approved by the NCI-funded oncology cooperative group chairs and was administered through a Web-based platform. A link to the survey was distributed via email to all members of the ECOG-ACRIN Cancer Research Group, Alliance for Clinical Trials in Oncology (Alliance) and Southwest Oncology Group (SWOG) by the ECOG-ACRIN Clinical Education and Awareness Team on 6/21/2017 with 3 subsequent weekly reminders. Responses were anonymous, and no incentives were provided to survey respondents. Descriptive statistics were used to analyze responses.
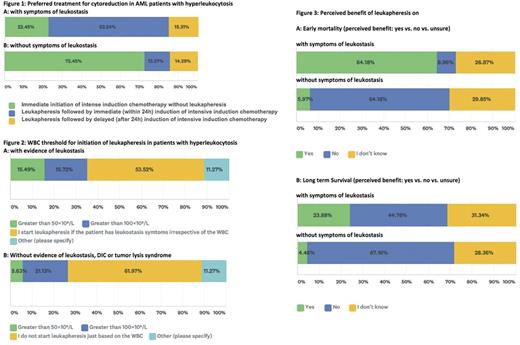
Results: There were 142 participants with a median age of 48 years (range, 30-75). Most responders (97%) were from North America representing 36 states. While 42% were clinicians, 26%, 23%, 2% and 6% of participants identified themselves as clinical researchers, research coordinators, basic scientists and others, respectively. Practice site included: 50%, 32%, 13% and 6% university hospital, community hospital, private practice, and other settings, respectively. Most responders (45%) defined hyperleukocytosis in acute myeloid leukemia as a white blood cell count (WBC) >100x109/L, while 18% and 4% of responders used a WBC of >50 x109/L and >30 x109/L as the cut-off; 23% did not indicate a specific threshold. Most responders (82%) indicated they would use leukapheresis in some patients. The responders who did not use leukapheresis most commonly cited lack of availability of the procedure at their facility (48%) or lack of perceived benefit (20%) as the primary reason; 6% cited unacceptable time required to consult transfusion medicine, while 32% cited other reasons. The majority of responders (77%) would use clinical evidence of leukostasis to initiate leukapheresis [Figure 1A]; 72% would avoid using leukapheresis if symptoms of leukostasis were not present [Figure 1B]. Others stated they did not decide based on signs of leukostasis but rather on WBC count [Figure 2A and B].
The majority (82%) did not indicate the upper age limit for using leukapheresis; 10% used 80 years as a cut-off. Most participants believed acute promyelocytic leukemia (62%) and hemodynamic instability (72%) to be contraindications, whereas only 20%, 30% and 40% of participants considered significant coronary artery disease, significant heart failure and disseminated intravascular coagulation (DIC) to be contraindications, respectively. Focusing on thrombocytopenia, 25%, 46%, 21% and 8% cited a platelet count >10, >20, >30 and >50 x 109/L as the minimal threshold required before initiating leukapheresis. For patients with severe symptomatic anemia and hyperleukocytosis, 41% did not transfuse red blood cells (RBCs) in patients with leukostasis (regardless of the WBC count) whereas 27% use a WBC in considering RBCs transfusions; 32% of responders would transfuse RBCs regardless of the presence of hyperleukocytosis or leukostasis. Only 18% of participants had guidelines available at their institutions when and how to use leukapheresis, while 60% indicated it was solely the attending physician's decision. Most responders felt that leukapheresis only had a beneficial effect on early mortality if leukostasis symptoms were present; however, about one third of responders were not sure whether leukapheresis influenced early mortality in patients with or without leukostasis [Figure 3A]. When asked about long-term survival, 67% of responders did not believe there is a benefit with leukapheresis in the absence of leukostasis; about one third of responders were unsure [Figure 3B].
Conclusions: This survey demonstrates significant variability in the definition and management of hyperleukocytosis and perceptions regarding indications and contraindications of leukapheresis. The findings emphasize the need for randomized clinical trials to define the role of leukapheresis in the management of hyperleukocytosis.
Stone: Celgene: Consultancy; Astellas: Consultancy; Jazz: Consultancy; Fuji Film: Consultancy; Arog: Consultancy; Agios: Consultancy; Abbvie: Consultancy; Sumitomo: Consultancy; Amgen: Consultancy; Pfizer: Consultancy; Ono: Consultancy; Novartis: Consultancy. Erba: Juno: Other: all research support paid to University of Alabama, Research Funding; Seattle Genetics: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Sunesis: Consultancy; Millennium/Takeda: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Glycomimetics: Other: Chair, Data and Safety Monitoring Committee; Agios: Other: all research support paid to University of Alabama, Research Funding; Celgene: Consultancy, Other: Chair, Scientific Steering Committee , Speakers Bureau; Incyte: all research support paid to University of Alabama, Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; Daiichi Sankyo: Consultancy, Other: all research support paid to University of Alabama, Research Funding; ImmunoGen: Consultancy, Other: all research support paid to University of Alabama, Research Funding; MacroGen: Consultancy; Ono: Consultancy; Pfizer: Consultancy; Astellas: Other: all research support paid to University of Alabama, Research Funding; Celator: Other: all research support paid to University of Alabama, Research Funding; Janssen: Other: all research support paid to University of Alabama, Research Funding. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees. Steensma: Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Janssen: Consultancy, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees; Onconova: Consultancy; Incyte: Equity Ownership; Celgene: Consultancy; Takeda: Consultancy; H3 Biosciences: Consultancy. Komrokji: Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau. Zeidan: AbbVie, Otsuka, Pfizer, Gilead, Celgene, Ariad, Incyte: Consultancy, Honoraria; Takeda: Speakers Bureau; Otsuka: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal